Pinpointing PFAS: The Sensor Breakthrough We Need
New sensor technologies are emerging. How close are we to rapid, reliable testing for PFAS?
We're living in a world increasingly aware of a vast family of synthetic compounds: Per- and Polyfluoroalkyl Substances, or PFAS. Numbering nearly 15,000 distinct chemical entities, their unique properties, stemming from the incredibly strong carbon-fluorine bond, have made them ubiquitous in industrial processes and consumer products for decades. Think of everything from specialized firefighting foams that save lives to the coatings that make our cookware non-stick, our carpets stain-resistant, and our food packaging grease-proof. But this utility comes at a steep price. Their very stability means they don't easily break down in the environment, leading to widespread contamination of water, soil, and air. They travel, they persist, and they can accumulate in living organisms, including us. The health concerns are significant and growing, with links to altered metabolism, increased cancer risks, and immune system disruptions.
As regulatory bodies finally begin to set stringent limits for some PFAS in drinking water – we're talking parts-per-trillion (ppt), an almost unimaginably tiny concentration – the demand for effective, accessible detection has become a critical public health and environmental priority. The dream is a simple, affordable, and reliable sensor, something anyone could use to get rapid insights about the water they drink or the environment they live in. Unfortunately, the path from this vision to a widely available, market-ready sensor is a marathon, not a sprint, paved with considerable scientific and practical roadblocks.
Why Detecting PFAS Is So Darn Hard?
Traditional methods for water quality monitoring, primarily laboratory-based liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS), are the current gold standard. These methods offer excellent sensitivity and specificity for individual PFAS compounds. However, they come with substantial per-sample analysis costs (often $200 to several hundred dollars), involve complex and time-consuming sample preparation, and require sophisticated, expensive instruments operated by highly trained personnel in controlled laboratory environments. This inherently limits their utility for widespread, rapid, or continuous on-site monitoring.
This is where sensor technology should offer a revolutionary alternative, providing potentially affordable, rapid solutions with unprecedented spatiotemporal resolution. But PFAS present a unique and formidable challenge for sensor developers.
Sensitivity: The new EPA limits of 4.0 ng/L (ppt) for PFOA and PFOS in drinking water, and even lower health advisory levels (e.g., 0.004 ppt for PFOA), set an incredibly demanding benchmark for sensor sensitivity. Achieving this, especially consistently in real-world samples, is a monumental hurdle. Many emerging sensors show promise in the lab, reporting detection limits in the parts-per-billion (ppb) to low ppt range using purified water or simple spiked samples. However, a significant "LOD gap" often exists – a disparity between these idealized lab results and what's reliably achievable when the sensors are faced with the chemical complexity of actual environmental samples. Furthermore, to boost apparent sensitivity, many highly sensitive detection schemes rely on preconcentration steps, such as solid-phase extraction (SPE). While effective, these steps add complexity, time, and potential for contamination, negating some of the primary advantages sensors aim to provide.
Selectivity: The term PFAS encompasses a vast and diverse family of compounds, varying in carbon chain length, the extent of fluorination, and the nature of their polar functional groups (like carboxylates or sulfonates). Most sensors are developed and optimized for one or just a few specific, often legacy, PFAS like PFOA or PFOS. Their ability to selectively detect these targets in the presence of hundreds or thousands of other PFAS congeners – or to respond predictably to a broader range of them – is often limited or not well characterized.
Beyond distinguishing between different PFAS, sensors must also contend with "matrix effects" – interference from a multitude of non-PFAS compounds present in environmental samples. Natural organic matter (like humic and fulvic acids), common inorganic ions (salts), proteins, lipids, and other pollutants can all wreak havoc. For instance, common anionic surfactants like sodium dodecyl sulfate (SDS) or sodium dodecylbenzenesulfonate (SDBS) can mimic PFAS behavior at sensor surfaces. Humic acids and chloride ions are known to interfere with certain electrochemical sensors. This chemical "noise" can cause false positive or false negative signals, distort quantitative measurements, or lead to sensor fouling, where the active surface becomes blocked or altered. Validating selectivity against this backdrop is a painstaking process.
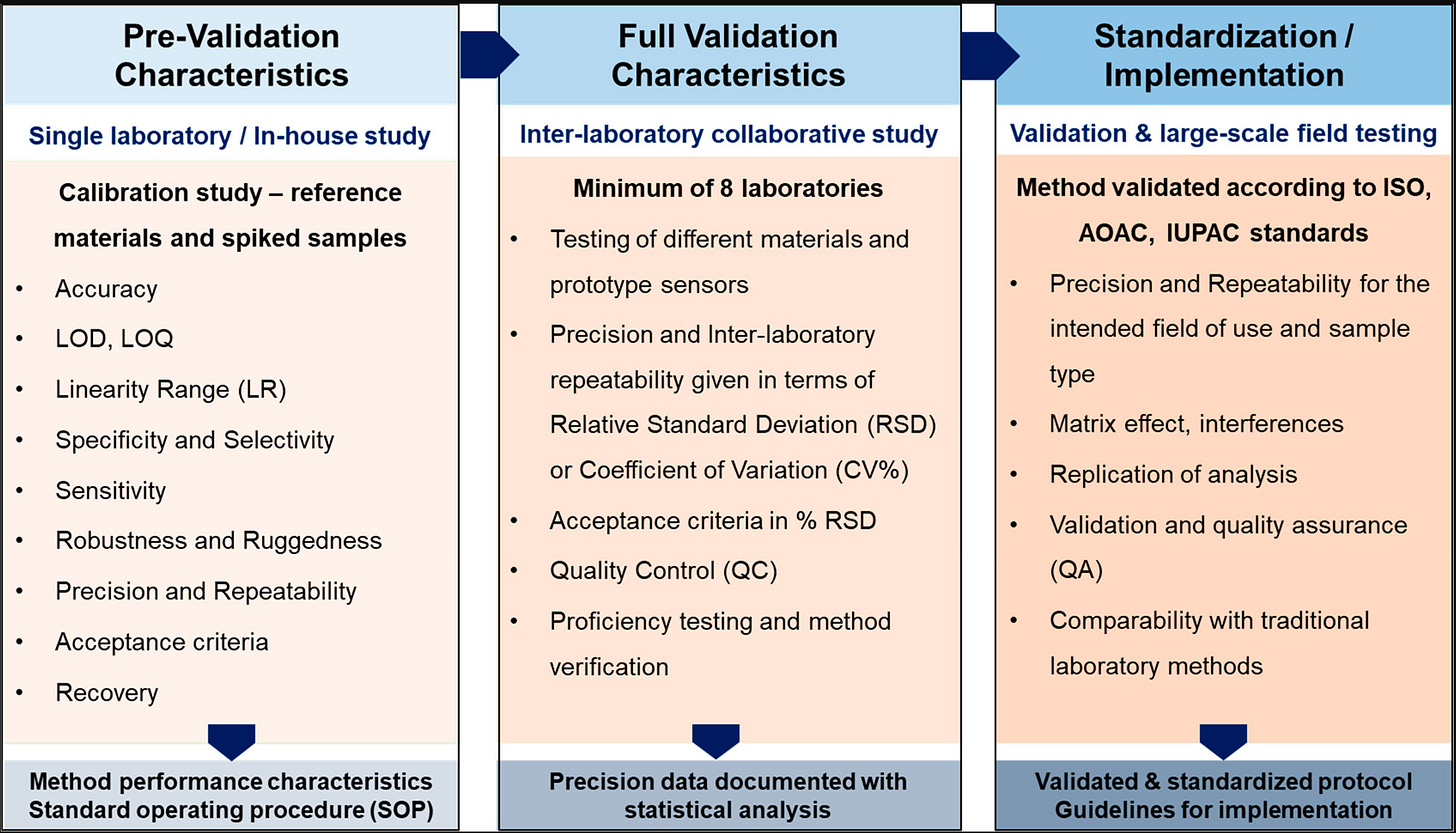
The Journey from Lab Bench to Field: The vast majority of academic research on sensors for emerging contaminants, including PFAS, currently sits at the early Technology Readiness Levels (TRLs 1-3). This typically means basic principles have been observed and a proof-of-concept prototype has been created, often guided by established working principles and subjected to preliminary performance evaluations in the lab.
Advancing to a commercial product involves much more than just good science. Phase 2 of development (TRLs 4-6) requires market research and customer discovery to assess actual needs and the potential market size. This leads to the development of a Minimum Viable Product (MVP) whose design and performance are refined based on customer feedback and rigorous evaluation and validation in relevant environments. Phase 3 (TRLs 7-9) focuses on demonstrating capabilities in intended operational environments, manufacturing individual and integrated components, and, finally, large-scale market implementation and commercialization.
This lab-to-field transition is where many promising technologies falter. A sensor must be robust enough to withstand harsh field conditions: temperature fluctuations, humidity, dust, vibrations, and potential exposure to corrosive substances, all without significant performance degradation. Power requirements for continuous or remote monitoring are a critical constraint. The need for reagents, calibration solutions, or disposable sensor cartridges in the field can limit practicality. And critically, background PFAS contamination from laboratory equipment, reagents, or even the air can compromise the validation of sensors targeting ultra-trace levels, leading to an overestimation of sensitivity. Many devices described as "portable" are still essentially smaller versions of laboratory instruments that demand considerable user expertise or elaborate sample pretreatment, limiting their practical utility for widespread field screening.
Glimmers of Innovation: Where's the Hope?
Despite these formidable challenges, the scientific community is not standing still. Ingenuity in materials science, nanotechnology, and biotechnology is paving the way for novel sensing strategies:
Electrochemical Sensors: These devices aim to detect PFAS by measuring changes in electrical properties (like current or potential) that occur when target analytes interact with a specially modified electrode surface. Since PFAS molecules themselves are generally not very electrochemically active, detection often relies on indirect mechanisms or highly specialized surface chemistry.
Molecularly Imprinted Polymers (MIPs) are a key strategy here. These are synthetic polymers created with custom-shaped cavities that selectively bind to target PFAS molecules, like a lock for a specific key. An AuNS-MIP composite on a glassy carbon electrode, for example, has been developed for PFOS detection, with some lab-based MIP sensors reporting LODs down to 7.5 ppt for PFOS or 1.7 ppt using ambient oxygen as a redox probe. Bare platinum electrodes have also been investigated for PFOS sensing based on its adsorption behavior.
Another approach involves micropipette-based interfaces between two immiscible electrolyte solutions (µITIES), which can detect the transfer of ionized PFAS across the liquid-liquid interface, though typically with LODs in the low ppb range.
Optical Sensors: These sensors operate by correlating changes in optical properties – such as absorbance, fluorescence, refractive index, or light scattering – with the concentration of PFAS.
Colorimetric sensors offer simplicity, producing a visible color change. For instance, a paper-based sensor using methylene green dye changes color in the presence of PFOS, though often with higher detection limits (e.g., 10 mg/L, or 10,000 ppb). Porphyrin-based detectors have also shown color changes with PFOA, but again at relatively high concentrations ( > 3 ppm).
Fluorescence sensors rely on changes in the light emitted by a fluorescent substance when it interacts with PFAS. Strategies include water-soluble fluorescent probes (cationic porphyrins, perylene diimide derivatives) whose fluorescence is quenched or enhanced by PFAS, with LODs reported from 3.3 ppb to 1350 ppb for PFOS. Amplifying Fluorescent Polymers or Conjugated Polymers (CPs) can shift their fluorescence spectra upon binding PFAS, with some reporting LODs as low as 0.08 ppb for PFOA.
Surface Plasmon Resonance (SPR) sensors are highly sensitive label-free detectors that measure minute changes in the refractive index at a metal surface when molecules bind. A notable advancement is an SPR sensor using a D-shaped plastic optical fiber coated with nanolayers of a MIP specific for PFAS, demonstrating an impressive LOD of 1.47 ppt for PFOA in diluted river water.
Surface-Enhanced Raman Spectroscopy (SERS) dramatically boosts the Raman scattering signal of molecules adsorbed on nanostructured noble metal surfaces (like silver or gold nanoparticles), allowing for highly sensitive and specific "fingerprinting" of molecules. A portable SERS sensor employing graphene and silver nanoparticles has reported LODs of approximately 0.5 ng/L (ppt) for PFOS and 70 ng/L for PFOA in lab water.
Whispering Gallery Mode (WGM) Microresonators, optical cavities that trap light, are also being explored for their high sensitivity to environmental changes, with detection down to 1 ppb demonstrated for PFAS using multiplexed systems.
Biosensors & Bio-inspired Approaches: These utilize biological recognition elements or bio-inspired systems coupled with a transducer.
Aptamers, short single-stranded DNA or RNA molecules selected to bind specific targets like PFOA with high affinity, are promising recognition elements. An aptamer-based sensor for PFOA using fluorescence transduction reported an LOD of 70.4 ppb and was tested in wastewater.
Specific proteins, like human liver fatty-acid-binding protein (hLFABP), have been modified with fluorophores to detect PFAS binding via changes in fluorescence, achieving LODs of 112 ppb for PFOA.
Even genetically engineered microorganisms, such as Pseudomonas aeruginosa, have been designed to produce a fluorescent signal in the presence of PFAS, demonstrating remarkable LODs as low as 10 ppt for PFOA/PFOS. However, this sensitivity often comes with a trade-off, such as a 24-hour incubation period to achieve maximum fluorescence, which may limit utility for rapid, on-site decisions.
An innovative and potentially very practical approach is the electrically-read Lateral Flow Assay (e-LFA). One such device uses a fluorous polyaniline (F-PANI) ink printed on a nitrocellulose membrane. Acidic PFAS molecules are selectively absorbed, doping the PANI and increasing its electrical conductivity. This system achieved LODs of 400 ppt for PFOA and 200 ppt for PFBA in tap water, offering potential for at-home water testing.
Novel Materials & Artificial Intelligence (AI): Underpinning many of these advancements is the relentless progress in materials science. Nanomaterials like graphene, quantum dots, gold nanostars, and carbon nanoarchitectures are used to increase electrode surface area, improve electron transfer, and provide platforms for recognition elements. Metal-Organic Frameworks (MOFs), with their highly tailorable porous structures, are being designed as "tunable capture probes". AI and Machine Learning (ML) are also playing an increasingly vital role, from accelerating the discovery of new sensing materials (e.g., by screening known materials to predict their efficacy as chemical probes for PFAS) to analyzing complex sensor data, enhancing signals, and improving quantification accuracy in noisy environments.
So, When Can I Test My Water Easily? The Path Forward
The hard truth is, while innovation is vibrant, we are not yet at the point of having a universally applicable, cheap, and foolproof PFAS field sensor that meets all regulatory demands for all situations. However, the intense research focus, driven by regulatory pressure and public concern, offers a clear path forward, though it requires concerted effort across multiple fronts:
Prioritize Real-World Performance over Lab Curiosities: Academic research, while crucial for fundamental breakthroughs, must increasingly pivot towards demonstrating robustness, reliability, and validated performance in complex, real-world environmental matrices – not just under pristine laboratory conditions. This means rigorous testing against certified reference methods (like EPA Method 1633) across diverse and representative sample types, and transparently reporting these findings.
Embrace True Stakeholder Collaboration & Co-Creation: Sensor developers must engage with end-users – including impacted communities, water utilities, environmental consultants, and regulatory agencies – from the earliest stages of design. Understanding their specific needs, practical constraints, and desired performance characteristics (e.g., sensitivity, selectivity, portability, cost, ease-of-use) is paramount. This co-creation process ensures that developed sensors are not just scientifically interesting but also genuinely useful and likely to be adopted.
Establish Standardization and Rigorous Validation Protocols: The development of universally accepted performance metrics and standardized testing protocols is crucial for the entire field. This would allow for objective comparison between different sensor technologies, build end-user confidence, and streamline the path towards regulatory acceptance. Promoting inter-laboratory comparison studies, potentially guided by organizations like ISO, AOAC, and IUPAC, is vital to validate protocols and ensure applicability across diverse institutions.
Strategic Funding and Partnerships for Translation: Increased public and private funding must be directed towards translational research – bridging that critical gap between laboratory discoveries and commercially viable products. Programs like the National Science Foundation’s Innovation Corps (I-Corps) and federal Small Business Innovation Research (SBIR) / Small Business Technology Transfer (STTR) programs are invaluable for supporting entrepreneurial activities, scaling-up, prototyping, and field validation. Academia-industry partnerships are essential for navigating the complex journey from prototype to market-ready device.
Develop an Interdisciplinary Workforce: Tackling the multifaceted challenges of PFAS sensor development requires a diverse range of expertise. We need to foster a workforce capable of working across disciplines – including analytical chemistry, materials science, biochemistry, engineering, data science, and even public policy and business development – through cross-disciplinary training, internships, and entrepreneurial opportunities.
Embrace a Tiered Monitoring Framework: It's unlikely that new sensors will immediately replace highly accurate laboratory methods for all purposes. Instead, a more practical approach is a tiered monitoring strategy. Rapid, lower-cost sensors could be deployed for initial field screening, site characterization, identifying contamination hotspots, or monitoring the effectiveness of remediation technologies in near real-time. This data can then guide the more targeted, efficient, and cost-effective use of conventional, high-precision laboratory methods for confirmatory analysis and regulatory compliance.
Focus on "Fit-for-Purpose" Solutions: While the pursuit of sub-ppt detection for all PFAS is a long-term ambition, sensors that can reliably detect broader classes of PFAS, or specific problematic ones at slightly higher but still environmentally or biologically relevant and actionable levels, could be immensely valuable now for certain applications. Tailoring solutions to meet the specific demands of various application scenarios, effectively balancing cost and performance, is key.
The quest for effective PFAS sensors is more than an academic exercise or a market opportunity; it's about developing vital tools to empower communities, inform regulators, and guide remediation efforts to manage a pervasive environmental challenge. The journey is complex and demanding. Yet, the convergence of material science, nanotechnology, biotechnology, and data science – fueled by a clear and urgent societal need – suggests that while a simple "PFAS dipstick" for all occasions might still be some way off, significant progress towards faster, more accessible, and field-deployable detection solutions is undeniably underway. It will require continued patience, persistent innovation, strategic investment, and a deeply collaborative spirit to translate these scientific breakthroughs into the practical tools needed to safeguard our health and our shared environment.
Links:
Sensors for Emerging Water Contaminants: Overcoming Roadblocks to Innovation: https://pubs.acs.org/doi/full/10.1021/acs.est.3c09889